 |
|||||||||||||||
Our studies involve using Cryptococcus neoformans as a model organism to study host-fungal interactions for the purpose of developing novel immune therapies and/or vaccines to treat or prevent invasive fungal infections. C. neoformans, the causative agent of cryptococcosis, is a fungal pathogen that frequently infects the central nervous system (CNS) of immune compromised individuals causing life-threatening meningoencephalitis. Exposure to C. neoformans via the inhalation of cryptococcal spores into the nasal passages is very common in the general population. Nevertheless, cryptococcal infections in the United States predominantly pose a significant health risk in immune compromised populations (i.e., individuals receiving corticosteroid therapy, individuals with lymphoproliferative disorders and organ transplant recipients). Studies have shown that 2.8% of organ transplant recipients can develop cryptococcal infections resulting in an overall death rate of 42%. In addition, the acute mortality rate is between 10-25% in medically-advanced countries and at least one third of patients with cryptococcal meningitis who receive appropriate therapy will undergo mycologic and/or clinical failure.
Cryptococcal meningoencephalitis is the most common disseminated fungal infection in AIDS patients. Those who are successfully treated for AIDS-associated cryptococcal meningitis oftentimes require life-long maintenance anti-fungal therapy due to a high relapse rate. Although, immune reconstitution due to highly active antiretroviral therapy (HAART) has been associated with a decrease in AIDS-associated cryptococcosis, the use of HAART to treat HIV infection has also been linked to the development of C. neoformans-related immune reconstitution inflammatory syndrome (IRIS) which is also life-threatening. A recent study found that 30% of HIV positive patients hospitalized for C. neoformans infection who received HAART subsequently developed IRIS. Therefore, there is an immediate need for therapies that are designed to treat or prevent C. neoformans infections. To this end, our current research efforts are focused in two areas.(1) To determine the mechanisms responsible for the development of protective immune responses against pulmonary C. neoformans infections. Our studies have shown that an experimental pulmonary infection with a genetically engineered interferon-gamma (IFN-g)-producing C. neoformans strain in mice results in the resolution of the acute infection and complete (100%) protection against a second pulmonary infection with a pathogenic C. neoformans strain (Figure 1). Based on these results, we are using this engineered strain in our current animal model system to determine the mechanisms that are involved in the induction of protective anti-cryptococcal host immune responses.
(2) To identify cryptococcal protein antigens to evaluate as vaccine candidates to combat C. neoformans infections. Using serum antibody obtained from mice that are protected against pulmonary C. neoformans infections, we will identify, clone, and characterize specific immune dominant cryptococcal proteins. Our objective is to evaluate the potential of these proteins to be used as vaccines for protection against future cryptococcal infections.
Figure 1. Survival in murine inhalational model. Mice received an initial inoculum of sterile PBS, 1 × 104 CFU of C. neoformans H99 IFN- g or heat-killed C. neoformans yeasts in 50 ml of sterile PBS, allowed 70 days to resolve the infection, and subsequently given a second challenge with 1 X 104 CFUof C. neoformans strain H99 in 50 ml of sterile PBS.
Survival of primary and secondary infected mice was monitored twice daily and mice that appeared moribund or not maintaining normal habits (grooming) were sacrificed by CO2 inhalation.
A B
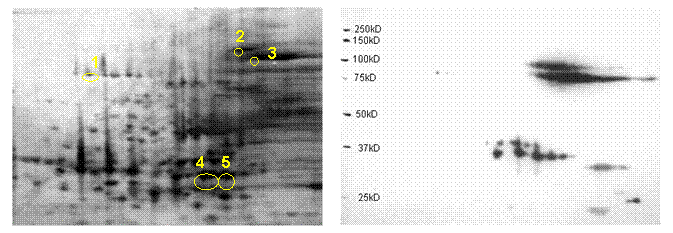
Figure 2. 2D SDS-PAGE profiles of a C. neoformans b-ME extract. A C. neoformans b-ME extract was prepared and resolved by IEF in the pH range 4¯7 and with a 10% gradient gel and stained with SYPRO Ruby Red (panel A), or prepared for Western blot analysis and staining with immune sera and eventual detection using a chemiluminescent substrate and silver (panel b). Samples 1-5 were subjected to proteolytic digestion and analyzed by nano liquid chromatography with tandem mass spectrometry (LC/MS/MS). MS/MS data was searched using a local copy of MASCOT (www.matrixscience.com) and peptide mass fingerprint data submitted to ProFound (Proteomics software package) for querying of NCBI database for peptide identification. Peptides 1-5 were found to be a putative heat-shock protein, heat-shock protein 90, aconitase, transaldolase, and carbamoyl-phosphate synthetase 1, respectively.
Publications
F.L. Wormley, Jr., J. R. Perfect, C. Steele, and G.M. Cox. (2006) Protection against cryptococcosis using a murine Interferon-gamma-producing Cryptococcus neoformans strain. Infection and Immunity. (manuscript in revision).
- F.L. Wormley Jr., M. Scott, W. Luo, M. Baker, J. Chabain, and PL. Fidel, Jr., (2000) Evidence for a unique CD4 protein on murine vaginal CD4+ T cells. Immunology. 100: 300-308.
- K.A. Kelly, H.L. Gray, J.C. Walker, R.G. Rank, F.L. Wormley Jr., and P.L. Fidel Jr., (2001) Chlamydia trachomatis infection does not enhance local cellular immunity against concurrent Candida vaginal infection. Infection and Immunity. 69(5): 3451-3454.
- F.L. Wormley Jr., J. Chaiban, and P.L. Fidel Jr., (2001) Cell adhesion molecule and lymphocyte activation marker expression during experimental vaginal candidiasis. Infection and Immunity. 69(8): 5072-5079.
- P.L. Fidel Jr., F.L. Wormley Jr., J. Chaiban, R.R. Chesson, and V. Lounev, (2001) Analysis of the CD4 protein on human vaginal T lymphocytes. American Journal of Reproductive Immunology. 45: 200-204.
- F.L. Wormley Jr., C. Steele, K. Wozniak, K. Fujihashi, J.R. McGhee, and P.L. Fidel Jr., (2001) Resistance of TCR d chain deficient mice to experimental Candida vaginitis. Infection and Immunity. 69(11): 7162-7164.
- J.E. Leigh, C. Steele, F.L. Wormley Jr., and P.L. Fidel Jr., (2002) Salivary cytokine profiles in the immunocompetent individual with Candida associated denture stomatitis. Oral Microbiology and Immunology. 17(5): 311-314.
- L. Cardenas-Freytag, C. Steele, F.L. Wormley Jr., E. Cheng, J.D. Clements and P.L. Fidel Jr., (2002) Partial protection against experimental vaginal candidiasis after mucosal vaccination with heat-killed Candida albicans and the mucosal adjuvant LT(R192G). Medical Mycology. 40(3): 291-299.
- K.L. Wozniak, F.L. Wormley Jr., P.L. Fidel Jr., (2002) Candida-specific antibodies during experimental vaginal candidiasis in mice. Infection and Immunity. 70(10): 5790-5799.
- F.L. Wormley Jr., J. Cutright, and P.L. Fidel Jr., (2003) Multiple experimental designs to evaluate the role of T-cell-mediated immunity against experimental vaginal Candida albicans infection. Med Mycol. 41(5): 401-9.
- J.R. Blankenship, F.L. Wormley Jr., M.K. Boyce, W.A. Schell, S.G. Filler, J.R. Perfect, J. Heitman, (2003) Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryotic Cell. 2(3): 422-30.
- C. Onyewu, F. L. Wormley, Jr., J. R. Perfect, and J. Heitman, (2004) Calcineurin target Crz1 functions in azole-tolerance but is not required for virulence of Candida albicans. Infection and Immunity 72(12): 7330-7333.
- F.L. Wormley, Jr., G.M. Cox, and J. R. Perfect. (2005) Evaluation of host immune responses to pulmonary cryptococcosis using a temperature-sensitive C. neoformans Calcineurin A mutant strain. Microbial Pathogenesis 38: 113-123.
- F.L. Wormley Jr. and J.R. Perfect. (2005) Immunology of infection owing to Cryptococcus neoformans, in Antifungal Agents: Methods and Protocols (E.J. Ernst and P.D. Rogers, eds.), Humana, Totowa, NJ.
- F.L. Wormley, Jr., G. Heinrich, J.L. Miller, J. R. Perfect, and G.M. Cox. (2005) Identification and characterization of an SKN7 homologue in Cryptococcus neoformans. Infection and Immunity 73(8).
 |
Links provided from Health Science Center pages to other web sites do not constitute or imply an endorsement of those sites, their content, or products and services associated with those sites. |